Research Articles
LAMP vs nPCR vs RT-PCR: A Comprehensive Sensitivity Comparison for Molecular Diagnostics
This article provides a systematic comparison of the sensitivity, specificity, and practical application of Loop-Mediated Isothermal Amplification (LAMP), nested PCR (nPCR), and real-time PCR (RT-PCR) for researchers and drug development...
Unveiling the Viral Universe: How Metagenomic Sequencing is Revolutionizing Virus Discovery and Pandemic Preparedness
Metagenomic next-generation sequencing (mNGS) is transforming viral discovery by enabling the unbiased detection and characterization of known and novel viruses directly from clinical and environmental samples.
Antigen vs. Nucleic Acid Tests: A Performance Analysis for Diagnostic and Therapeutic Development
This article provides a comprehensive analysis for researchers and drug development professionals on the performance characteristics, appropriate applications, and limitations of viral antigen tests and nucleic acid amplification tests (NAATs).
Unveiling the Viral Universe: Exploring Biodiversity, Discovery Methods, and Therapeutic Potential of Undiscovered Viruses
This article synthesizes current advancements and challenges in the exploration of viral biodiversity, a field revolutionized by metagenomics and artificial intelligence.
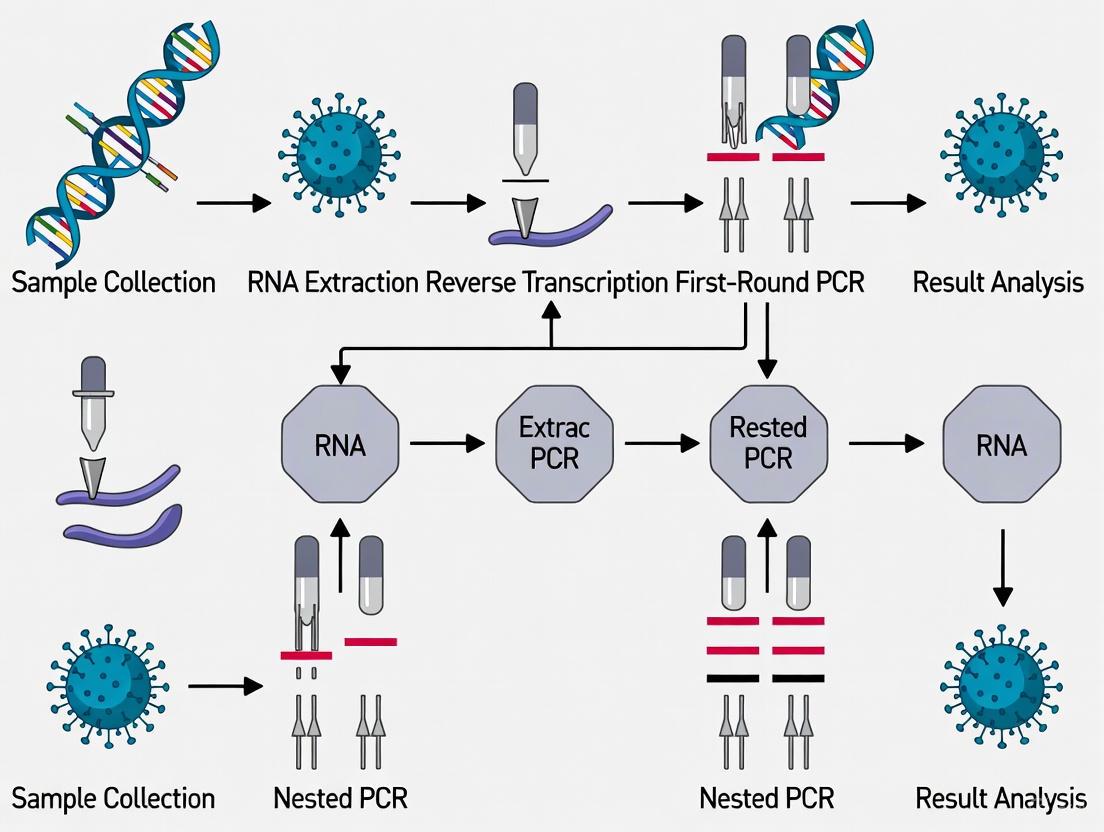
Nested PCR for SARS-CoV-2: A High-Sensitivity Detection Strategy for Research and Clinical Diagnostics
This article provides a comprehensive analysis of nested PCR assays for the detection of SARS-CoV-2, addressing the critical need for highly sensitive diagnostic tools in biomedical research and therapeutic development.
Viral Structure and Life Cycle Analysis: From Pathogen Architecture to Therapeutic Intervention
This article provides a comprehensive analysis of viral structure and replication cycles, tailored for researchers, scientists, and drug development professionals.
Predicting the Jump: Decoding Viral Zoonotic Potential and Cross-Species Transmission
This article synthesizes current research on the mechanisms and predictors of viral zoonotic potential for a scientific audience of researchers and drug development professionals.
Viral Load Quantification Methods: Correlation, Challenges, and Clinical Applications in Modern Biomedicine
This article provides a comprehensive analysis of viral load quantification methodologies, a cornerstone of clinical virology and therapeutic monitoring.
A Comprehensive Guide to Validating Home-Brewed NAT Assays: From Principles to Practice
This article provides a systematic framework for researchers, scientists, and drug development professionals on the validation of laboratory-developed nucleic acid amplification tests (NATs).
Navigating the Viral Phylogenetics Toolbox: A Comparative Guide for Biomedical Research
This article provides a comprehensive comparison of viral phylogenetic analysis tools, tailored for researchers, scientists, and drug development professionals.